

The rationale behind this is that the negative charge of the lone pair is more spread out in space, and tends to repel the bonding pairs (which are more localized, being more constrained to the region between the two bonded atoms), thereby pushing them to be closer together in space. This ideal tetrahedral angle will hold when four atoms of the same type are bonded to the central carbon atom, but when we replace one of the bonding electron pairs with a nonbonding lone pair, the bond angles for the remaining bonds tend to shrink from the ideal value. The bond angles for methane are indeed 109.5° - this is the angle made by any two C-H bonds (look at H at 12 o'clock and 8 o'clock - these are the bonds in the plane of the figure above).

Out of the plane of the page, and dashed wedges suggest bonds that Solid wedges are meant to suggest bonds pointed The sketches include the lone pairs (shown as balloons attached Of the three types of molecular structure that are based on a tetrahedralĪrrangement of electron domains around.
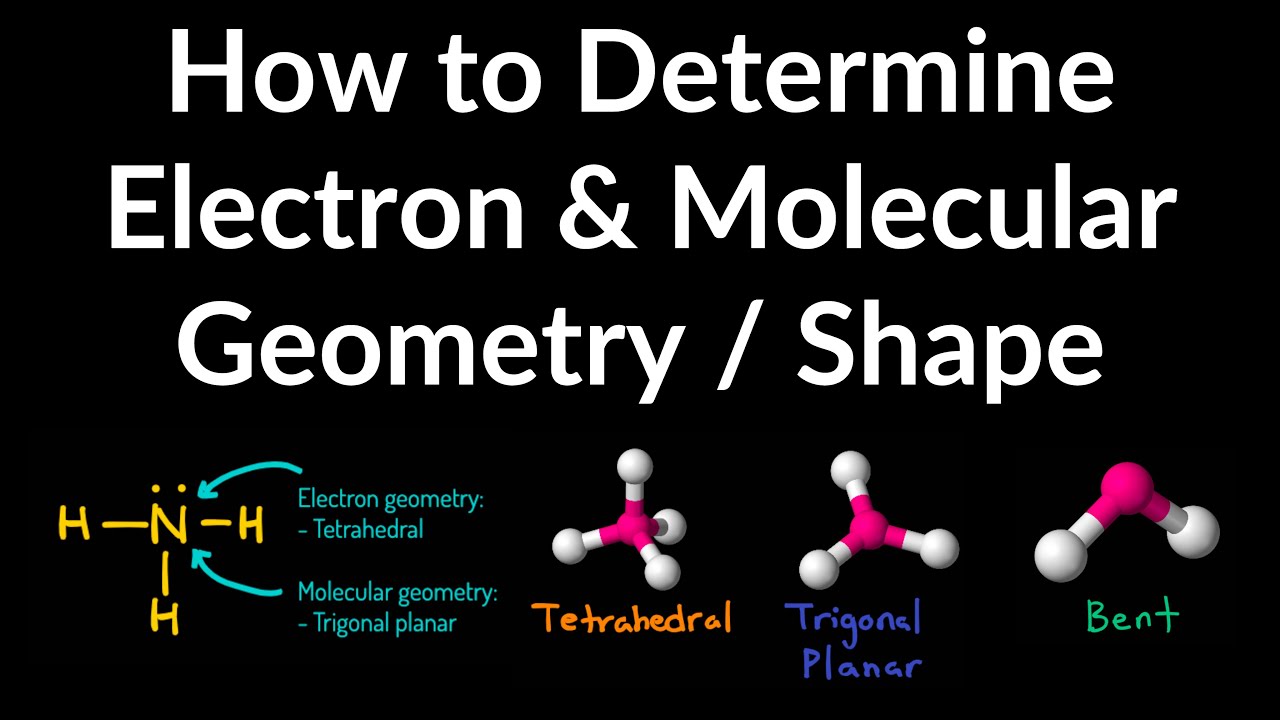
Representing three-dimensional molecules on a two-dimensional The ideal bond angles for this geometry are 109.5°.Īs an example of the tetrahedral structure,the figure at left shows the bond geometry of methane

If the SN = 4, the observed structures are based on the tetrahedron.įor the case with all four electron domains bonding (then thereĪre four atoms bonded to the central atom), the positions of theīound atoms lie at the corners of a tetrahedron. Groups (as are all the examples mentioned above) this results in an ideal bond angle of 120°. For the case in which all electron groups are bonding He VSEPR model predicts a trigonal planar geometry, with electron groups oriented toward theĬorners of an equilateral triangle. The organic molecule formaldehyde (CH 2O), The VSEPR model predicts a linear geometry and aĮxamples of trigonal planar geometry (SN = 3) are provided by boron trichloride (BCl 3), That is, in each case they count as one electron group, and In the latter both count the same in determining the steric number SN (i.e. Note that the single bonds in the former and the double bonds Note that any atom obeying the octet rule will not have SN > 4.Īnd we'll begin by analyzing the possible molecular shapes for steric number equal to 2, 3 and 4.Įxamples of linear geometry (SN = 2)are provided by beryllium dichloride (BeCl 2) andĬarbon dioxide (CO 2). The value we assign to SN is the same as the number of electron domains (electron groups). (Tro's "electron groups") instead of electron pair in determining a value for SN. Or triple-bonded to the central atom, we sometimes use the term electron domain Since we end up counting a bonded atom only once, even if it is double. Tro's text refers to this count, what we are calling steric number, The number of lone pairs present on the central atom. This amounts simply to a count of the number of bonded atoms plus Number (abbreviated SN) for the central atom of a molecular structure. To do this, we apply the valence shell electron pair repulsion or VSEPR model. Shape of the molecule, or its three-dimensional structure. Molecular structure: The five basic shapesįor simple molecules, we can use them to predict the Single-central atom structures: The five basic shapes. GENERAL CHEMISTRY TOPICS Molecular structure


 0 kommentar(er)
0 kommentar(er)
